

Gavi® Automated Vitrification
Vitrification is the most up to date and effective method of freezing oocytes and embryos, regardless of stage1. Talk to embryologists about manual vitrification and they will readily agree that the process can be tedious and time-consuming. There is a long learning curve, skills need to be maintained and intense focus is required by the embryologist.
Gavi®, the world’s first automated vitrification instrument, was designed by embryologists who wanted better control over the variables involved in manual vitrification – temperature, embryo handling, cryoprotectant concentrations and balancing equilibration/vitrification timing. Their aim was stage specific, controlled vitrification performed in a standardised way every single time – and they were successful.
Ideal Vitrification Environment
Gavi® helps you achieve optimal vitrification conditions in a different way12. It vitrifies oocytes, cleavage and blastocyst stage embryos with an automated, standardised, controlled protocol allowing vitrification to happen the same way every time12.
Oocytes/embryos are held in the Gavi® pod, key to the systems success the specially designed device also acts as the container for the oocytes/embryos in LN2 storage. Gavi® can vitrify 8 oocytes and cleavage stage embryos simultaneously (2 per pod) and 4 blastocysts simultaneously (1 per pod) allowing the embryologist to take control of other duties while vitrification is being performed. Gavi® helps you with:
- Automated solution dispensing: A robotic liquid-handling unit dispenses the necessary solutions with a precision difficult to achieve by hand, and eliminating the need to manually move embryos between fluids3.
- Gavi® Pod design: Designed to secure embryos in place during fluid exchange and liquid nitrogen (LN2) storage3, and to allow for fast cooling and warming rates.
- Control of exposure: The exposure timing to cryoprotectant solutions is carefully controlled to avoid toxic over-exposure3.
- Temperature control: sensors indicate that Gavi® is outside the recommended normal operational temperature range and if the heating module in Gavi® is outside of its acceptable temperature range12.
- Reduces the risk of cross-contamination: After equilibration, the Pod is automatically heat-sealed so it can be placed directly into LN212.
Gavi® Clinical Outcomes: The proof is in the results
At Genea, our manual vitrification outcomes were proven, data published in 2014 showed our single embryo transfer of vitrified-warmed blastocysts had equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers2. Gavi® needed to be as good as our current system – and it was.
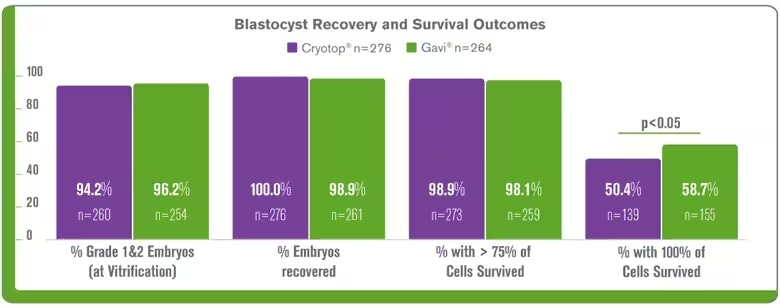
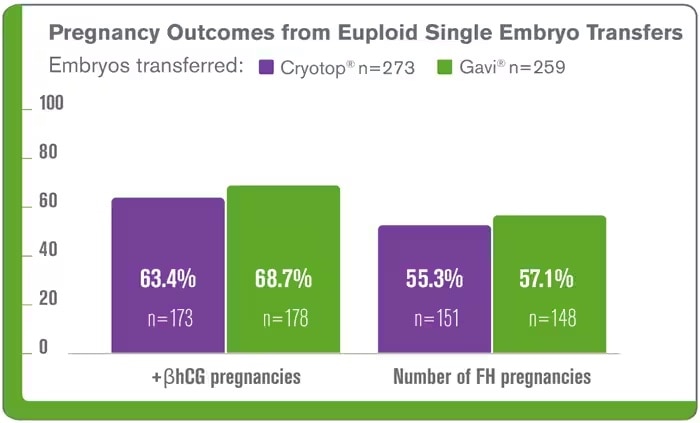
The following clinical data was obtained from the Genea clinic Gavi® implementation, it demonstrates that Gavi® delivers comparable clinical outcomes of blastocysts vitrified using the Gavi® system compared to Cyrotop system including, embryo survival and clinical pregnancy3,9 The results continue for Gavi®, it has been highlighted at conferences since its launch at ESHRE London 2013. ASPIRE5 (Brisbane, 2014), ALPHA6 (Antalya, 2014) and data from using Gavi® clinically for oocyte vitrification was presented at ASRM 2017 (O-136)7.
Easy to use regardless of experience
Any implementation period gives an opportunity to analyse data and so we looked back. It became obvious how easy Gavi® was to learn and how quickly embryologists were able to achieve benchmark results compared to the manual method8.
When analysing the early stage implementation data for the two techniques in Genea clinics we found using Gavi® there was a reduction in the variability of results between embryologists and the period between basic vitrification competency to aspirational competency was reduced8.
What about storage?
Any new approach needs to be adaptable, Gavi® pods are securely stored on cassettes in specially designed single or double level liquid nitrogen Gavi® Storage Dividers12. The dividers come in square or round configurations to fit easily into your existing storage vessels.
Gavi® Technology
- Pod with microfluidic dynamics that locates the embryo to allow automated fluid exchange
- Thin pod walls to ensure fast vitrification and warming rates
- Automated sealing to ensure pod is “fully closed” to eliminate risk of LN2 contamination
- Gavi® has been recognised internationally and awarded Gold in the Medical Design Excellence Awards in New York in 2017.
- Gavi® has been awarded the Australian Good Design Award in the product design category in 2017.
Get Instant Access
Enter your information below to view this resource.
"*" indicates required fields
Are you a Hamilton Thorne distributor?
Sign in to the distributor portal to gain access to all gated assets and exclusive content.
You may also be looking for
Case Studies
How other labs have utilized Hamilton Thorne solutions

New Mexico Cryobank and Andrology Lab (Roosters)

Zangersheide N. V. (Equine)

SafePharm Laboratories Limited (Rat)
1 Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C (2016). Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update. 23(2): 139-155.
2 Roy, T.K. et al., (2014). Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil. Steril, 101:1294-1301.
3 Roy, T. K., et al., (2013). “Automated closed vitrification instrument delivers equivalent outcomes to manual open vitrification for embryos.” Oral at 3rd International Congress on Controversies in Cryopreservation of Stem Cells, Reproductive Cells, Tissue and Organs (CRYO), Berlin, Germany.
4 Roy, T.K., et al., (2013). “Novel automated instrument executing a standardised closed vitrification protocol yields equivalent outcomes with human blastocysts when compared with open manual vitrification system.” Human Reproduction, 28 (Suppl 1): i62-i64
5 Accepted for poster presentation at ASPIRE, The 5th Congress of the Asia Pacific Initiative on Reproduction, Brisbane, Australia 2014
6 Accepted for poster presentation at ALPHA, The 10th Biennial Conference for Scientists in Reproductive Medicine, Antalya, Turkey, 2014
7 Sole M, Polyzos N, Gonzalez Llagostera C, Carrasco B, Coroleu B, Veiga A, Boada M (2017). Automatic vs manual vitrification of human oocytes. Preliminary results of the first randomised controlled trial using sibling oocytes. Fertil. Steril. 108(3) (Supplement): e57.
8 Alpha Scientists in Reproductive Medicine (2012). The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod. Biomed. Online 25: 146-167.
9 Tumurbaatar U, Hobson N, Roy TK, McArthur S (2017). Introduction of a semi-automated vitrification system reduces variability between embryologists and delivers comparable laboratory and clinical outcomes. (white paper)
10 Tucker, et al. Vitrification in Assisted Reproduction, Second Edition. CRC Press.2015.
11 Roy TK, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Human Reproduction. 2014;19(11):2431–2438.
12 QFRM168 Gavi user manual



